Date:2025-07-30
Supercritical foaming is currently quite popular in footwear applications. Today, let's take a deeper look at this process. This article will cover an introduction to supercritical foaming, a comparison of different foaming agents, a comparison with chemical foaming, common footwear processes, and industry players. Before reading, please join the footwear industry group for discussion.
I. Introduction to Supercritical Foaming
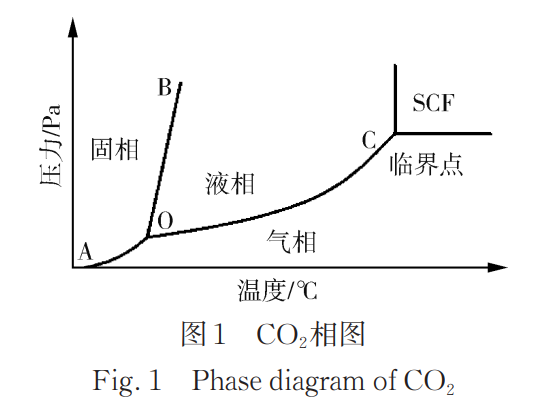
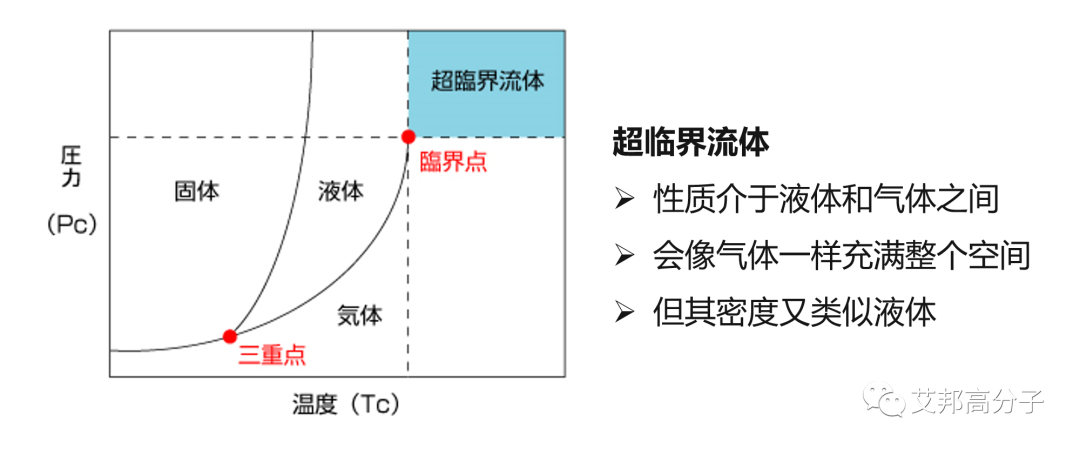
Supercritical fluids (SCFs) are fluids whose pressure and temperature are both above their critical point.
In the CO2 phase diagram (Figure 1), point O represents the triple point, where three phases coexist in equilibrium. Point C is the endpoint of the gas-liquid equilibrium line, known as the critical point. The temperature and pressure at this point are the critical temperature and critical pressure, respectively.
SCFs retain the properties of a gas while also exhibiting liquid-like properties. Their diffusion coefficient is 10 to 100 times that of liquids. Due to their excellent permeability, flow, heat transfer, and mass transfer properties, they have a strong solubility for many substances and can extract and separate non-volatile and heat-sensitive substances at relatively low temperatures. For example, supercritical carbon dioxide (scCO2) is CO2 at a temperature and pressure above its critical temperature of 31.1°C and critical pressure of 7.38 MPa. At this point, CO2 possesses the properties of both a gas and a liquid, improving mass transfer performance.

Popular Shoe Material Process - Supercritical Foaming
Comparison of Different Foaming Agents
Foaming Agent Type Advantages Disadvantages
CO2 has a high solubility and diffusion rate in polymers. Under the same conditions, CO2 produces more bubbles and a lower product density. Its diffusion coefficient differs significantly from that of air, resulting in more significant product shrinkage than nitrogen.
N2 has a similar diffusion coefficient to air, resulting in product dimensions that are stable and less prone to shrinkage. Its low diffusion rate and solubility in polymers result in longer foaming times or require higher saturation pressures, resulting in reduced production efficiency and increased equipment investment costs.
II. Comparison of Supercritical Foaming and Chemical Foaming
Supercritical foaming: Gases such as carbon dioxide and nitrogen are treated at high temperature and pressure to form a supercritical fluid, which acts as a foaming agent. The process of converting a supercritical fluid into a gas at room temperature and pressure is a physical change. Popular Shoe Material Process - Supercritical Foaming
Features of Supercritical Foaming:
Supercritical foaming produces pure foam materials with food-safe grades and good skin compatibility.
Compared to chemical foaming, supercritical foaming has a finer cell structure and more stable performance.
Supercritical foaming foams have greater impact strength, better thermal stability, toughness, good sound insulation, and lower thermal conductivity and thermal conductivity.
Long saturation times can affect production efficiency, and rapid heating or pressure release places high demands on energy and equipment safety.

Popular Shoe Material Process - Supercritical Foaming Diagram
Supercritical Sheet Foaming Process
Chemical Foaming: Chemical foaming agents such as azodicarbonamide and sodium bicarbonate. For example, azodicarbonamide (also known as AC foaming agent) decomposes upon heating to produce nitrogen, carbon monoxide, carbon dioxide, and ammonia. This process is a chemical reaction. Popular Shoe Material Process - Supercritical Foaming
Chemical foaming (using azodicarbonamide as an example) features:
Highest gas evolution, relatively uniform pores, superior performance, and a wide range of applications.
The decomposition temperature is adjustable, without affecting curing and molding speeds, making the process highly mature.
AC foaming agent is a yellow crystal, and decomposition tends to produce a large number of byproducts. For example, insufficiently fine particle size can lead to a high number of byproducts, resulting in a product with an odor and color deviation.
Popular Shoe Material Process - Supercritical Foaming Diagram: EVA Sheet Chemical Foaming Process
III. Common Supercritical Foaming Processes in Shoe Material Applications
Currently, common supercritical foaming processes in the shoe material industry include: beads, sheets, and small-to-large foaming (injection molding + compression molding). Generally speaking, they all share the following common features:
They fully utilize the rapid diffusion rate and high solubility of supercritical fluids in polymers.
During foaming, the polymer is in a semi-solid state. The molten zone allows for cell growth, while the unmelted zone provides melt strength and maintains the cell structure. Extremely rapid pressure relief induces an extremely high nucleation rate, ensuring the formation of a micro-nano pore structure with a high pore density.
Popular footwear process—Supercritical foaming solid-state foaming mechanism
1. Supercritical fluid bead foaming process
Bead foaming can be categorized into two types: autoclave and extrusion. Unless otherwise specified, the more common autoclave method will be used as an example. The simplified process flow for bead foaming is as follows:
Polymer particles are placed in an autoclave;
A supercritical fluid is introduced, which swells and diffuses into the polymer matrix under high temperature and pressure;
The pressure is then rapidly released through a pressure relief valve to achieve microcellular foaming.
Common supercritical foamed particles, such as ETPU, are also known as popcorn. After obtaining the supercritical foamed particles, they undergo a steam molding process to form the final shoe sole.
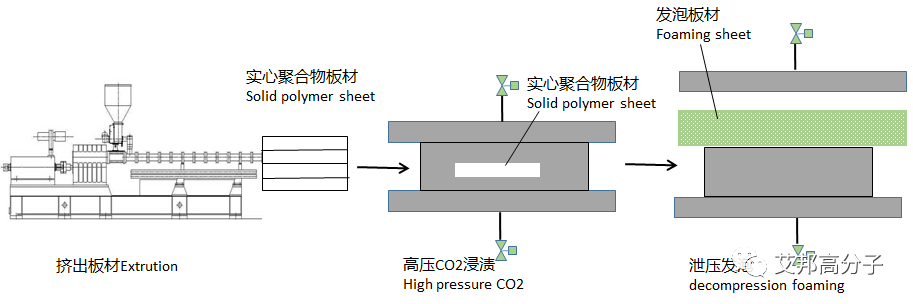
2. Supercritical Fluid Sheet Foaming Process
The supercritical sheet foaming process can be performed using either an autoclave or a press process. Here is a brief description of the press process:
The foaming mold on the press is heated.
Once the foaming temperature is reached, the polymer is placed in the mold, the press is closed, and the mold is sealed.
Supercritical fluid is then injected into the mold, allowing it to swell and diffuse into the polymer for 30 to 180 minutes.
The press is then opened to release pressure and foam, resulting in a polymer microcellular foam sheet with controllable cell size and density.
The resulting supercritical foam sheet is then cut to form a shoe sole.
3. Small-to-Big (Injection Molding + Supercritical Fluid Foaming)
Small-to-big, also known as mold foaming, is characterized by the small mold becoming larger after foaming. The process is as follows:
The materials are mixed;
The mold is cleaned and injection molded to produce a small sole mold;
The sole mold is then placed in the mold and foamed with supercritical fluid to produce the larger sole.
